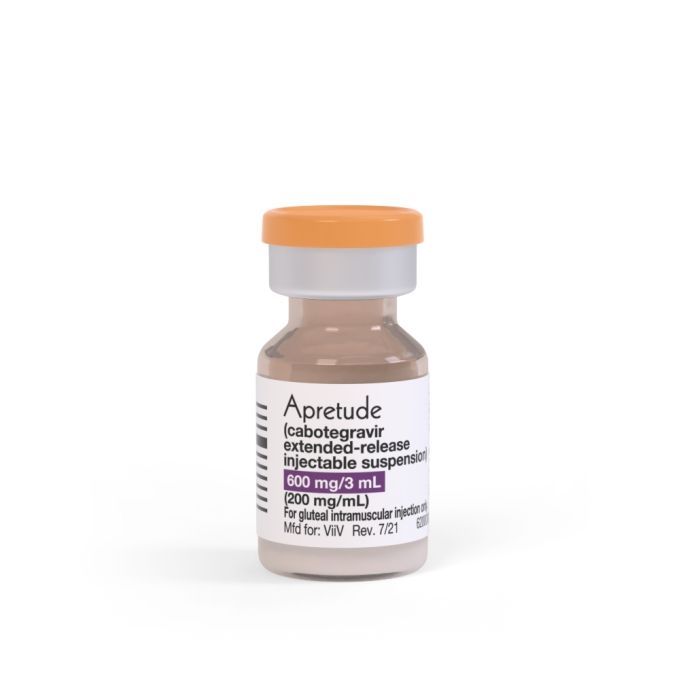
Apretude (cabotegravir extended-release injectable suspension)
$8,538.48
Apretude (cabotegravir extended-release injectable suspension)
What is Apretude (cabotegravir extended-release injectable suspension) for?
Apretude (cabotegravir extended-release injectable suspension) is a HIV-1 integrase strand transfer inhibitor (INSTI) indicated as pre-exposure prophylaxis (PrEP) option to reduce the risk of sexually acquired HIV-1. It is for use in adults and children weighing at least 35 kg who are at risk of sexually acquiring HIV and who have a negative HIV-1 test prior to start of treatment.[1]
It is available in suspension form for injection into a muscle, each containing 600 mg/3 mL (200 mg/mL) cabotegravir extended-release injectable suspension.[1]
How does Apretude (cabotegravir extended-release injectable suspension) work?
The active ingredient in Apretude, cabotegravir, is an integrase strand transfer inhibitor (INSTI). INSTIs can block an enzyme called integrase, which the virus uses to replicate in the body. By blocking integrase, cabotegravir lowers the number of HIV particles in the blood and keeps the levels low.[1,2]
The medicine cannot cure HIV infection or AIDS, but it can allow the immune system to repair itself and prevent further damage.[1,2]
Where has Apretude (cabotegravir extended-release injectable suspension) been approved?
Apretude (cabotegravir extended-release injectable suspension) was approved for HIV PrEP by:[3]
- The Food and Drug Administration (FDA), USA on December 20, 2021.
The FDA granted Apretude with Priority Review and Breakthrough Therapy designation.
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
How is Apretude (cabotegravir extended-release injectable suspension) taken?
The standard dosage is:[1]
- Initial dosage: 600 mg given 1 time every month for the first 2 months
- Thereafter: 600 mg is given 1 time every 2 months
Apretude (cabotegravir extended-release injectable suspension) is given into the muscle of your buttock (intramuscular injection).[1]
It is strongly recommended that you adhere to the injection schedule. The injections after the initial dosage may be given up to 7 days before or after the scheduled date.[1]
Before you receive your first injection of Apretude, your treating doctor may have you take cabotegravir tablets once daily for 1 month, to assess how well you tolerate the drug.[1]
Complete information about Apretude (cabotegravir extended-release injectable suspension) dosage and administration can be found in the official prescribing information listed in our references section.[1]
Note: Please consult with your treating doctor for personalised dosing.
Are there any known adverse reactions or side effects of Apretude (cabotegravir extended-release injectable suspension)?
Common adverse reactions
The most common side effects (≥1% of patients) listed in the prescribing information include:[1]
- Injection site reactions
- Diarrhea
- Headache
- Pyrexia
- Fatigue
- Sleep disorders
- Nausea
- Dizziness
- Flatulence
- Abdominal pain
- Vomiting
- Myalgia
- Rash
- Decreased appetite
- Somnolence
- Back pain
- Upper respiratory tract infection
Serious adverse reactions
The serious adverse reactions listed in the prescribing information include:[1]
- Allergic reactions
- Liver problems
- Depression or mood changes
Use in a specific population
Before you start Apretude (cabotegravir extended-release injectable suspension) treatment, tell your treating doctor about all your medical conditions, including if you:
- Have ever had a skin rash or an allergic reaction to medicines that contain cabotegravir
- Have or have had liver problems
- Have ever had mental health problems.
- Are pregnant or plan to become pregnant. It is not yet known whether Apretude can harm your unborn child. The drug can remain in your body for up to 12 months or longer after the last injection. Tell your treating doctor if you become pregnant while receiving Apretude.
- Are breastfeeding or plan to breastfeed. It is not known if Apretude can pass to your baby in your breast milk.
For a comprehensive list of side effects and adverse reactions please refer to the official prescribing information.[1]
References
1. Full prescribing information [FDA]: Apretude (cabotegravir extended-release injectable suspension) [PDF]
ViiV Healthcare, Dec 2021
2. Cabotegravir
NIH’s Office of AIDS Research, cited Jan 2022
| One kit containing 600 mg/3 mL single-dose (200 mg/mL) cabotegravir | 8,538.48 |
|---|
Be the first to review “Apretude (cabotegravir extended-release injectable suspension)” Cancel reply
Related products
HIV/AIDS
HIV/AIDS
HIV/AIDS
HIV/AIDS
HIV/AIDS






Reviews
There are no reviews yet.