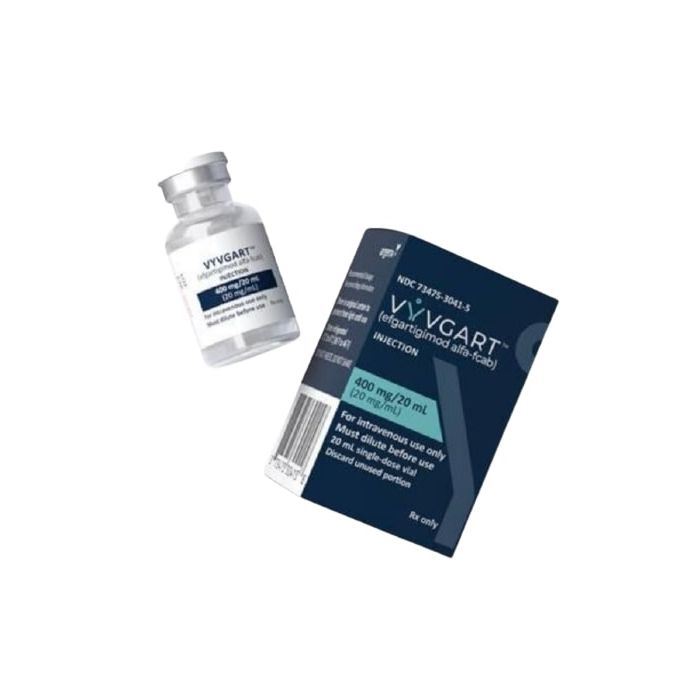
Vyvgart (efgartigimod alfa-fcab)
$13,184.24
Buy Vyvgart (efgartigimod alfa-fcab)
What is Vyvgart (efgartigimod alfa-fcab) for?
Vyvgart (efgartigimod alfa-fcab) is a neonatal Fc receptor blocker used to treat generalized Myasthenia Gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody positive.[1]
It is available in vial form for intravenous infusion, each containing 20 mg/mL efgartigimod alfa-fcab.[1]
How does Vyvgart (efgartigimod alfa-fcab) work?
Myasthenia gravis (MG) is a rare, chronic, neuromuscular disorder. It causes weakness in skeletal muscles (voluntary muscles) of the whole body. With normal muscle function, nerve impulses originating in the brain travel through nerves towards the muscle fibers. At the junction between the nerve and the muscle, a chemical called acetylcholine is released, which is used to communicate.[2]
In MG, the body’s immune system makes antibodies against acetylcholine receptors (AChR) present at this junction, which results in muscle weakness.[2]
Vyvgart is designed to lower levels of these antibodies. It does so by blocking the activity of the protein neonatal Fc receptor (FcRn), which normally helps protect certain antibodies from being broken down.[3]
Where has Vyvgart (efgartigimod alfa-fcab) been approved?
Vyvgart (efgartigimod alfa-fcab) was approved for the treatment of people with gMG by:
- The Food and Drug Administration (FDA), USA on December 17, 2021.[4]
- The Pharmaceuticals and Medical Devices Agency (PMDA), Japan on January 20, 2022.[5]
Vyvgart (efgartigimod alfa-fcab) was granted Fast Track and Orphan Drug designations by the FDA.[4]
A decision on Vyvgart’s approval in Europe is expected by mid-2022. [3]
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
How is Vyvgart (efgartigimod alfa-fcab) taken?
The standard dosage is:[1]
- 10 mg/kg given as an intravenous infusion (drip) over one hour once weekly for 4 weeks
- In patients weighing 120 kg or more: 1200 mg (3 vials) per infusion
Subsequent treatment cycles should be given based on clinical evaluation. The safety of starting a new treatment cycle sooner than 50 days from the start of the previous cycle has not been established.[1]
If you miss a scheduled infusion, you may be given Vyvgart up to 3 days after the scheduled time point. Thereafter, resume the original dosing schedule until the treatment cycle is completed.[1]
Complete information about Vyvgart (efgartigimod alfa-fcab) dosage (preparation) and administration can be found in the official prescribing information listed in our references section.[1]
Note: Please consult with your treating doctor for personalised dosing.
Are there any known adverse reactions or side effects of Vyvgart (efgartigimod alfa-fcab)?
Common adverse reactions
The most common side effects (≥10% of patients) listed in the prescribing information include:[1]
- Respiratory tract infections
- Headache
- Urinary tract infection
Serious adverse reactions
The serious adverse reactions listed in the prescribing information include:[1]
- Infection
- Undesirable immune reactions (hypersensitivity reactions
Use in a specific population
Before starting with treatment, tell your doctor about all of your medical conditions, including if you:[1]
- Have a history of infection or you think you have an infection
- Plan to receive any vaccines. Immunization with live-attenuated or live vaccines is not recommended during treatment with Vyvgart
- Are pregnant or planning to be pregnant and are breastfeeding or plan to breastfeed
For a comprehensive list of side effects and adverse reactions please refer to the official prescribing information.[1]
References
1. Full prescribing information [FDA]: Vyvgart (efgartigimod alfa-fcab) [PDF] Argenx, Dec 2021
2. What is Myasthenia Gravis?
Myasthenia.org, cited Dec 2021
3. FDA Approves Vyvgart for Adults With Most Common Form of gMG
Myastheniagravisnews.com, Dec 2021
4. FDA Approves New Treatment for Myasthenia Gravis
FDA press release, Dec 2021
5. argenx announces VYVGART™ approval in Japan for the treatment of generalized myasthenia gravis
Argenx press release, Jan 2022
| One vial of 400 mg/20 mL (20 mg/mL) | 13,184.24 |
|---|
Be the first to review “Vyvgart (efgartigimod alfa-fcab)” Cancel reply
Related products
Alzheimer's disease
Multiple Sclerosis (MS)
Alzheimer's disease
Multiple Sclerosis (MS)
Multiple Sclerosis (MS)
Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic Lateral Sclerosis (ALS)
Multiple Sclerosis (MS)







Reviews
There are no reviews yet.