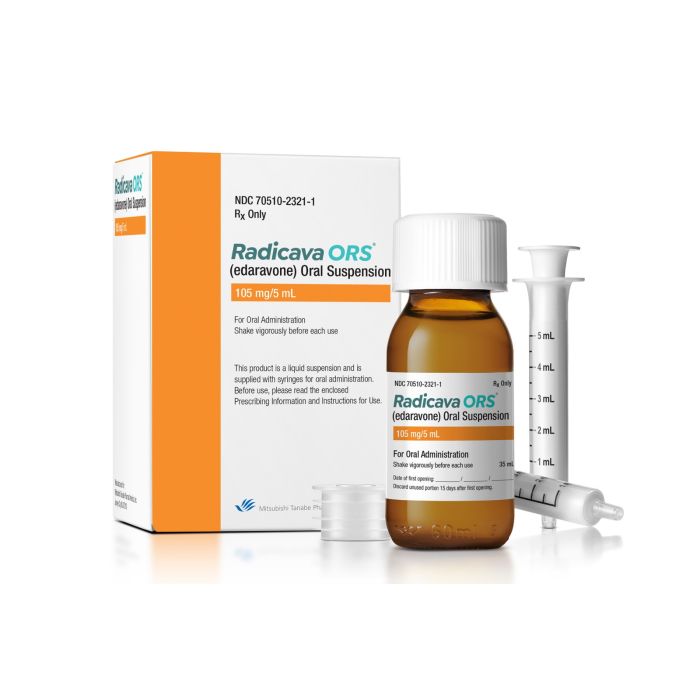
Radicava ORS (edaravone)
Radicava ORS (edaravone) is an oral medication for the treatment of patients with amyotrophic lateral sclerosis (ALS).
Radicava ORS is an oral formulation of edaravone, which is also available under as an intravenous formulation named Radicut. We have this medicine available to order as well. See [Radicut (edaravone)].
Buy Radicava ORS (edaravone)
What is Radicava ORS (edaravone) for?
Radicava ORS (edaravone) is an oral medication used to treat patients with ALS.[1]
Radicava ORS (edaravone) is available as a bottle containing 35 mL (105 mg/5 mL) of edaravone for oral use.
How does Radicava ORS (edaravone) work?
Radicava ORS (edaravone) protects neurons from oxidative stress by scavenging free radicals that may cause cellular damage. This mechanism is expected to be effective in patients with ALS. [1]
Although the etiology of development and disease progress of amyotrophic lateral sclerosis (ALS) are unknown, a possible involvement of oxidative stress caused by free radicals is suggested [1]. The mechanism by which edaravone exerts its therapeutic effect in patients with ALS is unknown [8]. It is, however, expected to suppress the disease progression by exerting its inhibitory effects against the development of oxidative damage to nerve cells [1].
Where has Edaravone been approved?
On the 12th of May 2022, the Food and Drug Administration (FDA), USA, has approved Radicava ORS (edaravone) oral suspension for the treatment of adults with ALS. Radicava ORS is an orally administered version of Radicava, which was originally approved in 2017 as an intravenous (IV) infusion to treat ALS.
How is Radicava ORS (edaravone) taken?
The standard dosage is:[1]
- 105 mg (5 mL) taken orally or through a feeding tube. Radicava ORS has 2 different dosing schedules: one for the first cycle and one for all of the following cycles:
- First 28-day cycle: 14 consecutive days on, 14 consecutive days off
- All following 28-day cycles: 10 out of 14 days on, 14 consecutive days off
Radicava ORS is taken orally or through a feeding tube. See the official prescribing information for further details on how to properly take the medicine.[1]
Radicava ORS should be taken in the morning on an empty stomach after overnight fasting.[1]
Complete information about Radicava ORS (edaravone) dosage and administration can be found in our references section.[1]
Note: Please consult with your treating doctor for personalised dosing.
Are there any known side effects of Radicava ORS (edaravone)?
Common adverse reactions
The most common adverse reactions (≥10% of patients) listed in the prescribing information include:[1]
- Bruising (contusion)
- Problems walking (gait disturbance)
- Headache
Serious adverse reactions
The serious adverse reactions listed in the prescribing information include:[1]
- Hypersensitivity reactions
For a comprehensive list of side effects and adverse reactions please refer to the official prescribing information[1].
References
1. Summary of Product Characteristics [FDA]: Radicava ORS (edaravone) [PDF] Mitsubishi Tanabe Pharma Corporation, May 2022.
2. MT Pharma Press Release. MT Pharma Co. receives approval for additional indication for ALS in Japan.
26/06/2015.
3. Abe K., et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients.
Amyotroph Lateral Scler Frontotemporal Degener. 2014 Dec; 15(7–8):610–7
4. Yoshino H. and Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study).
Amyotroph Lateral Scler. 2006 Dec.; 7(4):241–5.
5. Public summary of opinion on orphan designation. [PDF] 23/02/2015.
6. Orphan Designation.
Cited on Jun. 2016.
7. FDA News Release: FDA approves drug to treat ALS,
05/05/2017.
8. Summary of Product Characteristics [FDA]: Radicava (edaravone) [PDF] MT Pharma America Inc., May 2017.
9. What is Radicava (edaravone)?
Cited on May 2017.
10. Summary of Product Characteristics [Health Canada]: Radicava (edaravone) [PDF] Mitsubishi Tanabe Pharma America, Inc., October 2018.
11. FDA approval of RADICAVA ORS® for the treatment of ALS
May 2022.
For more information please visit our blog page “What is Radicut/Radicava?”
| Starter Kit: 1 bottle of 35 mL (105 mg/5 mL dose) | 23,834.20 |
|---|
Be the first to review “Radicava ORS (edaravone)” Cancel reply
Related products
Migraine
Migraine
Duchenne Muscular Dystrophy
Migraine
Multiple Sclerosis (MS)
Multiple Sclerosis (MS)
Amyotrophic Lateral Sclerosis (ALS)
Multiple Sclerosis (MS)







Reviews
There are no reviews yet.