Aduhelm (aducanumab-avwa)
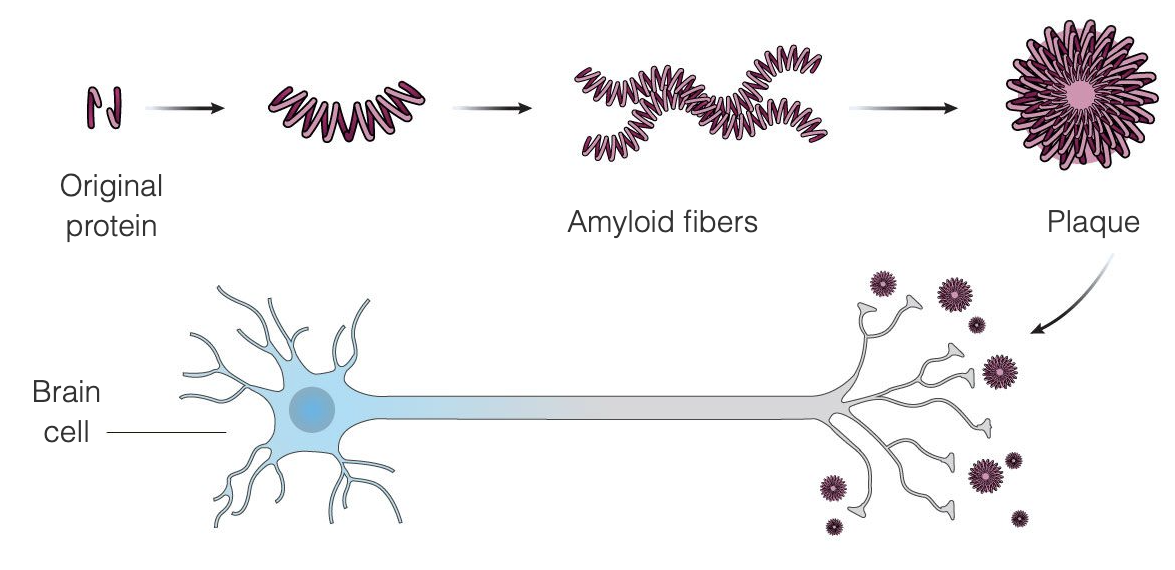
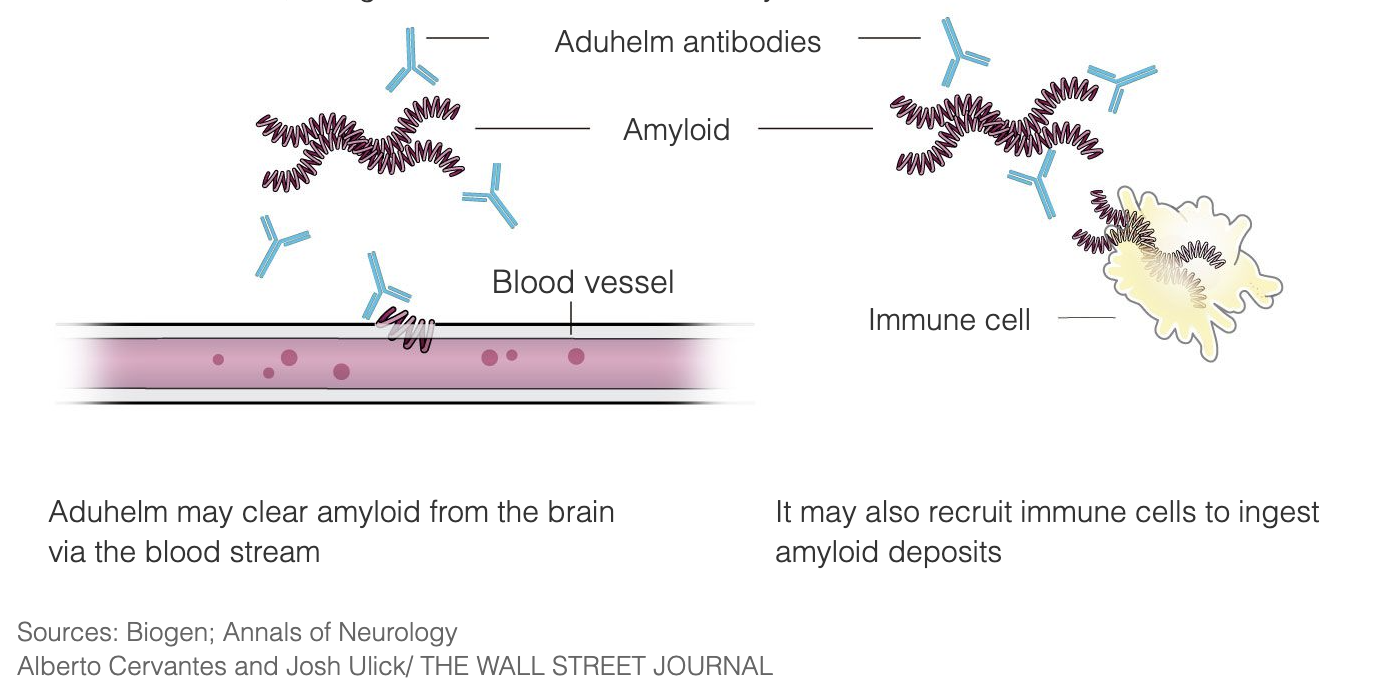
Aduhelm (aducanumab-avwa) is a prescription medicine used to treat people with mild cognitive impairment or mild dementia,. It is the first novel therapy approved for Alzheimer’s disease since 2003.
As of June 11 2021, everyone.org can help you access Aduhelm if you are outside of the United States. Make an enquiry below.
___________________
Please note that the FDA’s decision to approve Aduhelm has been criticised by some experts and that the results of the clinical trials are being challenged. We carry Aduhelm as part of our commitment to provide access for patients to any medicines as reviewed and approved by at least one national regulatory authority (such as the FDA) and only on the basis of a prescription from their doctor and under their supervision.
The doctor is – as with any other treatment – responsible for their patient’s treatment with Aduhelm (aducanumab) at every step. Read more in our post “What is Aduhelm (aducanumab)?“.





Reviews
There are no reviews yet.